Artimes™ Pro Clinical Trial Completed Successfully in U.S.
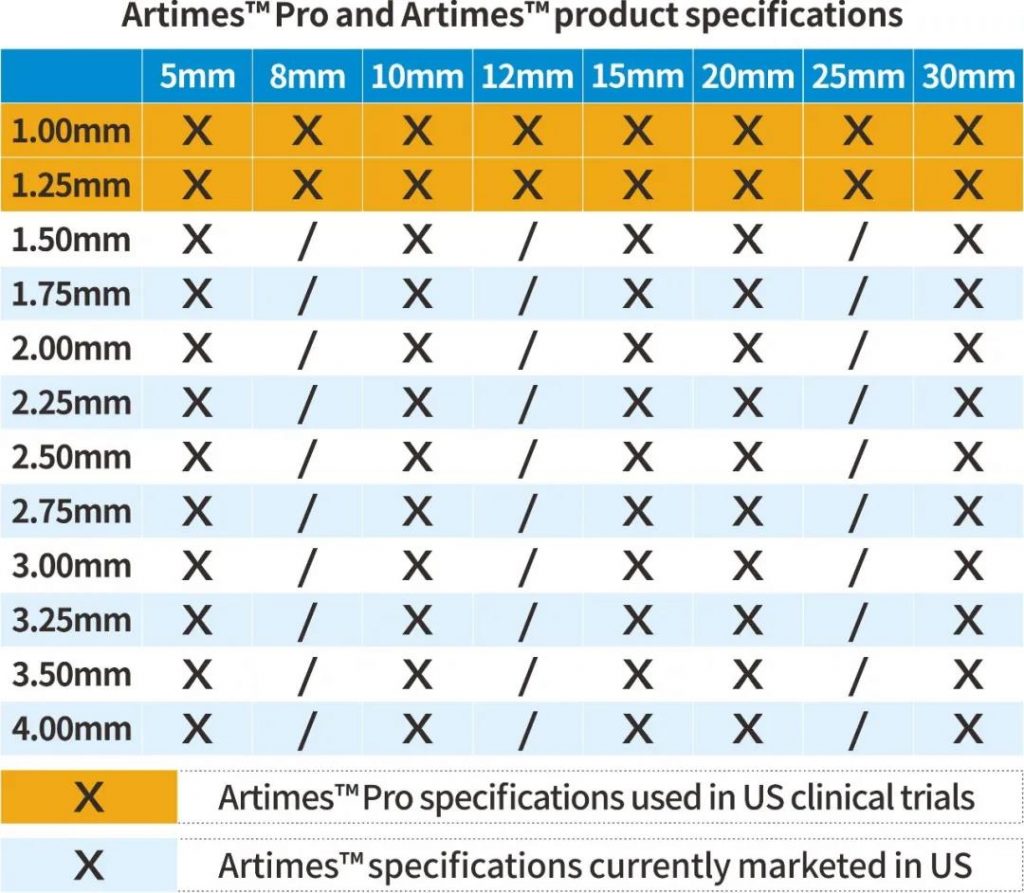
A clinical trial of Artimes™ Pro, a rapid exchange coronary balloon dilatation catheter manufactured by BrosMed Medical, has been successfully completed in the United States. The diameters of the balloon dilatation catheters are 1.0 mm and 1.25 mm. The crossing profile of 1.0mm balloon is ultra-small of 0.0218’’, which is suitable for CTO lesions.
The Artimes™ Pro clinical trial is a prospective, non-randomized, open clinical trial led by Dr. Jasvinder Singh of Washington University in the United States. All 60 patients in the trial presented with 70-100% coronary stenosis/CTO lesions. The ratio of 1.0mm and 1.25mm balloons used in the clinical trial was 3:1. The final procedural success rate reached 97.9%, and no adverse events related to the device occurred.
BroMed Medical has been deeply engaged in the field of coronary angioplasty for many years. The Artimes™ ( K141025, 1.5-4.0 mm) balloon portfolio has been sold in United States since 2015 and distributed in more than 50 countries around the world. Based on its proven manufacturing process and technology, Artimes™ Pro, a pre-dilatation PTCA balloon for stenotic CTO lesions, is estimated to get 510K clearance in 2021.